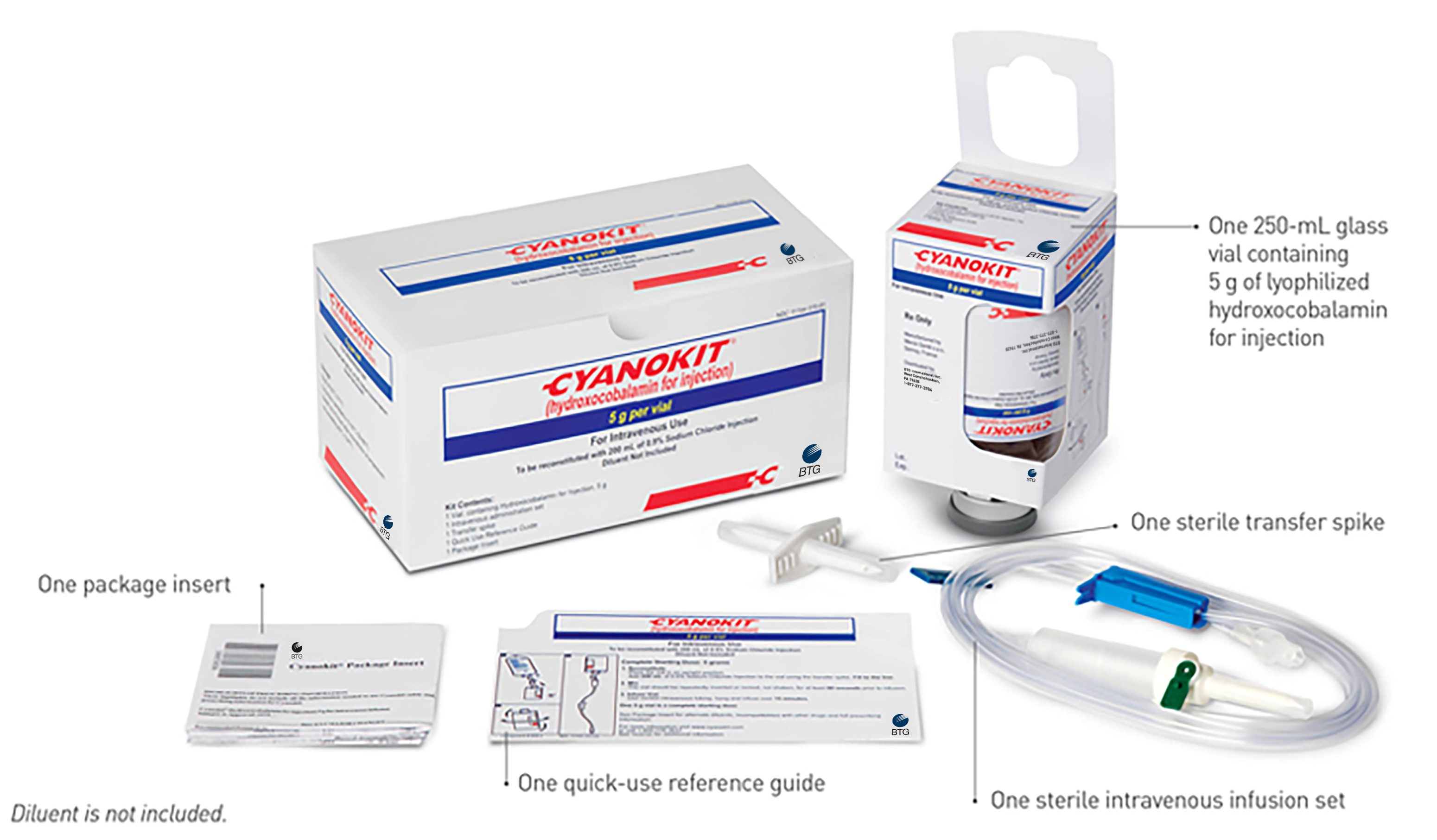
How to Order CYANOKIT
If you have questions about CYANOKIT, including how to order, request a demo kit, or other product-related questions, please contact the Specialty Solutions Center® at 1-844-293-0007 or email us at CYANOKIT@btgsp.com.
The Following Suppliers Are Authorized Distributors of CYANOKIT
AmerisourceBergen
| Henry Schein Inc.
|
Bound Tree Medical LLC
| Life-Assist Inc.
|
Cardinal Health
| McKesson Medical-Surgical
|
Dealmed Medical Supplies LLC
| McKesson Corporation
|
DMS Pharmaceutical Group Inc.
| Medline Industries, LP.
|
FFF Enterprises
| Morris & Dickson Co.
|
Hammer Medical
| RegiMed Medical
|
CYANOKIT Is the Preferred Agent to Be Stocked for Immediate Availability15
Two (2) CYANOKITs should be immediately available for administration on patient arrival15,†
- Antidotes such as CYANOKIT can be life-saving but must be available at the appropriate time to be effective.
- For treatment of cyanide toxicity, hydroxocobalamin was preferred over sodium nitrite and sodium thiosulfate because of its wider indications, ease of use, and anticipated safety in widespread use.
*Physical stocking location may vary by institution and by drug
preparation requirements.
†Prospective, nonrandomized, or
nonblinded clinical trials; cohort or well-designed case-control
studies; good-quality observational or volunteer studies.
SERB offers demo kits of CYANOKIT free of charge. If you are interested in a demo kit or have other questions regarding CYANOKIT, please contact us at cyanokit@btgsp.com.
| NDC | Carton Dimensions |
|---|---|
NDC 50633-310-11 | W: 194 mm x L: 100 mm x H: 97 mm |